
Chemistry, 14.05.2021 14:00, rubiim9610
What is the density of a gas at STP that has a molar mass of 50.0 g/mol?
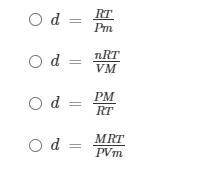
Student A wrote some preliminary notes: PV = nRT; n = m/M; and d = m/V
known: unknown
T = 273 K d = g/cm3
P = 1 atm
R = 0.08125 atm
M = 50.0 g/mol
If PV = nRT and n = m/M, what is the density of the ideal gas?
Help Student A find the equation to use to solve this problem:

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, jocelynmarquillo1
Acamcorder has a power rating of 17 watts. if the output voltage from its battery is 7 volts, what current does it use?units:
Answers: 1


Chemistry, 22.06.2019 13:00, monkeyrose1999
The molality of calcium chloride (cacl2) in an aqueous solution is 2.46 m. what is mole fraction of the solute?
Answers: 3
Do you know the correct answer?
What is the density of a gas at STP that has a molar mass of 50.0 g/mol?
Student A wrote some preli...
Questions in other subjects:


Biology, 20.08.2019 08:10


Mathematics, 20.08.2019 08:10

History, 20.08.2019 08:10

Mathematics, 20.08.2019 08:10

Mathematics, 20.08.2019 08:10



History, 20.08.2019 08:10






