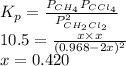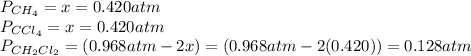Chemistry, 14.05.2021 03:00, karose4590
The equilibrium constant, Kp, for the following reaction is 10.5 at 350 K: 2CH2Cl2(g) CH4(g) CCl4(g) Calculate the equilibrium partial pressures of

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:30, UaRemomGAY
If anyone would be able to me out with these three questions it would be these are from the chem 2202 course.
Answers: 3


Chemistry, 22.06.2019 23:30, treylartigue
The appropriate concentration for an iodine sanitizer is
Answers: 1
Do you know the correct answer?
The equilibrium constant, Kp, for the following reaction is 10.5 at 350 K: 2CH2Cl2(g) CH4(g) CCl4(g)...
Questions in other subjects:

Mathematics, 21.01.2021 20:00

History, 21.01.2021 20:00


Mathematics, 21.01.2021 20:00


Mathematics, 21.01.2021 20:00

History, 21.01.2021 20:00

Mathematics, 21.01.2021 20:00


History, 21.01.2021 20:00


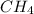 ,
, 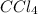 and
and 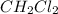 is 0.420 atm, 0.420 atm and 0.128 atm.
is 0.420 atm, 0.420 atm and 0.128 atm.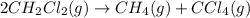
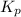 for this reaction is as follows.
for this reaction is as follows.