
Chemistry, 13.05.2021 22:20, angieplasencia8
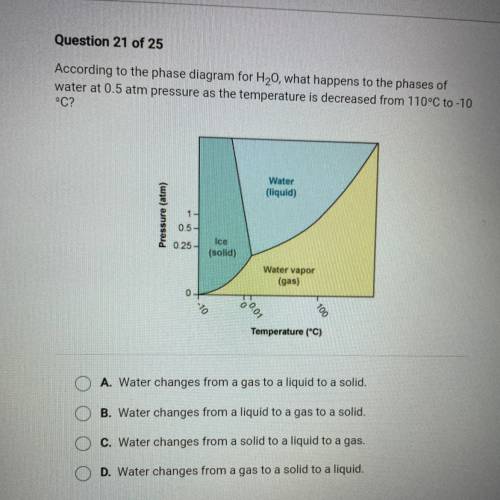
According to the phase diagram for H20, what happens to the phases of
water at 0.5 atm pressure as the temperature is decreased from 110°C to -10
°C?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, jonloya264
If 1.8 l of water is added to 2.5l of a 7.0 molarity koh solution, what is the molarity of the new solution
Answers: 1


Do you know the correct answer?
According to the phase diagram for H20, what happens to the phases of
water at 0.5 atm pressure as...
Questions in other subjects:


Computers and Technology, 22.02.2021 19:40




History, 22.02.2021 19:40


Health, 22.02.2021 19:40


History, 22.02.2021 19:40






