
Chemistry, 13.05.2021 22:00, mettababeeeee
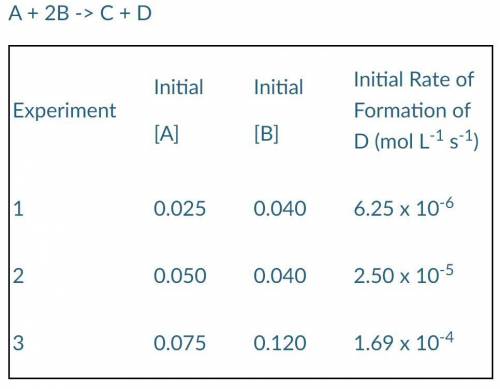
The following results were obtained when the reaction represented above was studied at 25°C:
A + 2B -> C + D
_
a) Determine the order of the reaction with respect to A and B.
b) Write the rate law for the reaction.
c) Calculate the value of the rate constant, k, specifying units.
d) Determine the initial rate of disappearance of substance B in Experiment 2.
_
e) Identify which of the reaction mechanisms represented below is consistent with the rate law developed in part B. Justify your choice by writing rate laws for each mechanism. Also, identify any intermediate(s) and/or catalyst(s) in your chosen mechanism. (6 pts - 1 pt for chosen mechanism, 1 pt for each rate law, 2 pts for intermediate(s)/catalyst(s))
_
Mechanism #1: A -> M (slow)
M + B -> C + X (fast)
X + B -> D (fast)
Mechanism #2: B ⇋ M (fast equilibrium)
M + A -> C + X (slow)
B + X -> D (fast)
Mechanism #3: A + B ⇋ M (fast equilibrium)
M + B -> C + X (slow)
X -> D (fast)
_

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, AvreeanaS1379
The first element on the periodic table of elements is carbon. a. true b. false
Answers: 2


Chemistry, 22.06.2019 19:30, Adrian12313
Estimate the molar mass of the gas that effuses at 1.6 times the effusion rate of carbon dioxide.
Answers: 1

Chemistry, 23.06.2019 07:30, bryantjorell
Which statement is actually true about the relationship between activation energy and reaction rates? low activation energy barriers result in low rates. high activation energy barriers result in low rates. low activation energy barriers result in no reaction. high activation energy barriers result in no reaction.
Answers: 2
Do you know the correct answer?
The following results were obtained when the reaction represented above was studied at 25°C:
A + 2B...
Questions in other subjects:

English, 23.03.2021 17:00

Biology, 23.03.2021 17:00


Chemistry, 23.03.2021 17:00

Mathematics, 23.03.2021 17:00

Mathematics, 23.03.2021 17:00

Mathematics, 23.03.2021 17:00


Mathematics, 23.03.2021 17:00

Mathematics, 23.03.2021 17:00






