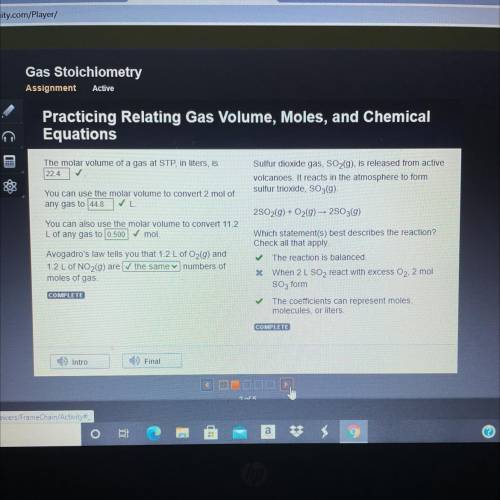
The molar volume of a gas at STP, in liters, is
22.4 ✓
You can use the molar volume to conver...

The molar volume of a gas at STP, in liters, is
22.4 ✓
You can use the molar volume to convert 2 mol of
any gas to 44.8
You can also use the molar volume to convert 11.2
L of any gas to 0.500
mol.
Avogadro's law tells you that 1.2 L of O2(9) and
1.2 L of NO2(9) are the same numbers of
moles of gas.
COMPLETE

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:00, officialgraciela67
What is the volume occupied by 10.0 dm3 of gas at standard pressure after it has been compressedat constant temputure to 500.0 kpa?
Answers: 1

Chemistry, 22.06.2019 10:40, rntaran2002
What is the ph of a 0.0010 m hno3? 1.0 3.0 4.0 5.0
Answers: 2

Chemistry, 22.06.2019 22:30, safiyabrowne7594
[ou.03jthe pictures below show the wavelengths and intensities of electromagnetic radiations emitted by three stars, star 1, star 2, and star 3. intensity intensity- intensity- 1000 3500 6000 8500 11000 wavelength (a) star 1 1000 3500 6000 8500 11000 1000 3500 6000 8500 11000 wavelength (a) wavelength (a) star 2 star 3 which of these statements is correct about the color of the three stars? star 2 is white in color o star 2 is yellow in color star 1 and star 3 are yellow in color star 1 and star 3 are white in color
Answers: 1

Chemistry, 23.06.2019 10:30, krlx
When a chemist collects hydrogen gas over water, she ends up with a mixture of hydrogen and water vapor in her collecting bottle. if the pressure in the collecting bottle is 97.1 kilopascals and the vapor pressure of the water is 3.2 kilopascals, what is the partial pressure of the hydrogen?
Answers: 1
Do you know the correct answer?
Questions in other subjects:


Mathematics, 18.10.2019 00:30





Biology, 18.10.2019 00:30








