
Chemistry, 13.05.2021 19:10, bethyboop1820
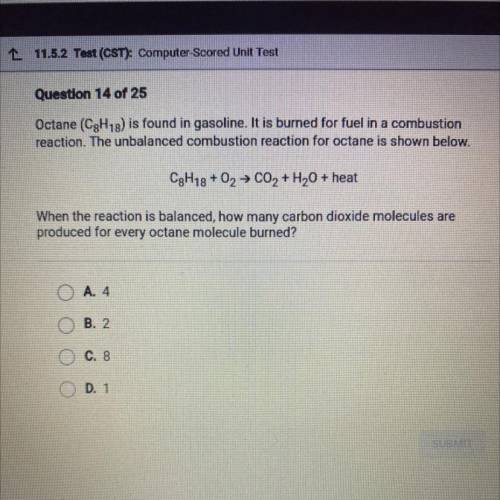
Octane (C8H18) is found in gasoline. It is burned for fuel in a combustion
reaction. The unbalanced combustion reaction for octane is shown below.
C8h18+ O2 → C02 + H2O + heat
When the reaction is balanced, how many carbon dioxide molecules are
produced for every octane molecule burned?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 15:30, abdullaketbi71
What best discribes the relationship between wavelength and frequency in a electromagnetic wave
Answers: 1

Chemistry, 22.06.2019 17:30, Naysa150724
Oil rich countries in the middle east cover about 4% of earths total land area but prossess about 48% of the worlds known oil reserves what is the main reason for high concentration of reserves in this part of the world
Answers: 3

Chemistry, 22.06.2019 18:30, tanviknawale
Which sample at stp has the same number of atoms as 18 liters of ne at stp
Answers: 1
Do you know the correct answer?
Octane (C8H18) is found in gasoline. It is burned for fuel in a combustion
reaction. The unbalanced...
Questions in other subjects:

Mathematics, 03.11.2019 08:31



Mathematics, 03.11.2019 08:31

Physics, 03.11.2019 08:31

Mathematics, 03.11.2019 08:31

Mathematics, 03.11.2019 08:31

Mathematics, 03.11.2019 08:31







