
Chemistry, 13.05.2021 18:10, Aracelys3946
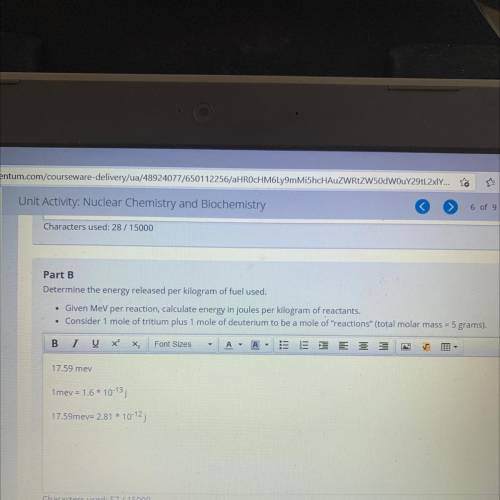
Part B
Determine the energy released per kilogram of fuel used.
• Given MeV per reaction, calculate energy in joules per kilogram of reactants.
• Consider 1 mole of tritium plus 1 mole of deuterium to be a mole of "reactions" (total molar mass = 5 grams).

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, AbhiramAkella
Plz choose one of the compounds from the table and explain how you know the numbers of atoms in your formula. is it possible for two different compounds to be made from the exact same two elements? why or why not? with a limited number of elements (less than 120 are known), does this mean we also have a small number of compounds or do we have a large number of compounds in this world?
Answers: 1

Chemistry, 22.06.2019 00:30, boonkgang6821
The clouds are grey and ground is wet. a quantitative b qualitative
Answers: 1

Chemistry, 22.06.2019 13:30, suemmimonjaras8374
The atomic number, or number, is the described as the number of in the nucleus of an chemical element.
Answers: 1
Do you know the correct answer?
Part B
Determine the energy released per kilogram of fuel used.
• Given MeV per reaction, cal...
• Given MeV per reaction, cal...
Questions in other subjects:

History, 09.03.2020 23:54

Physics, 09.03.2020 23:54

Mathematics, 09.03.2020 23:54

Geography, 09.03.2020 23:54











