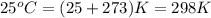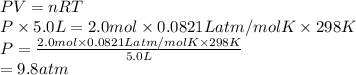Chemistry, 13.05.2021 09:40, laylac45531
A sample of 2.0 moles of helium gas is contained in a tank with a volume of 5.0L at a temperature of 25°C. What is the pressure of the gas in the tank
in atm?
Given: R = 0.0821 L. atm/mol. K
O 9.8 atm
O 0.00069 atm
O 0.82 atm
O 0.0082 atm

Answers: 3
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 22:00, huddyxo
Scientists often have to deal with numbers that are either very large or very small. for example, the radius of the sun is approximately 696,000 kilometers, while bacterial cells are as small as 1.9 × 10-4 millimeters. express each number in an alternate form.
Answers: 1

Chemistry, 22.06.2019 23:10, carmenguabaoql9kv
Afusion reaction takes place between carbon and another element. neutrons are released, and a different element is formed. the different element is a) lighter than helium. b)heavier than helium. c)the same weight as helium. d)dependent on the element that reacted with carbon.
Answers: 3
Do you know the correct answer?
A sample of 2.0 moles of helium gas is contained in a tank with a volume of 5.0L at a temperature of...
Questions in other subjects:

Mathematics, 02.08.2019 13:40

Mathematics, 02.08.2019 13:40


Mathematics, 02.08.2019 13:40

Mathematics, 02.08.2019 13:40




Biology, 02.08.2019 13:40

Mathematics, 02.08.2019 13:40