
Chemistry, 13.05.2021 04:30, cutielove2912
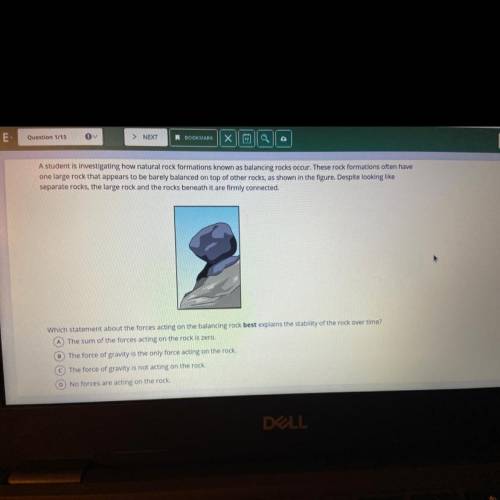
A student is investigating how natural rock formations known as balancing rocks occur. These rock formations often have one large rock that appears to be barely balanced on top of other rocks, as shown in the figure. Despite looking like separate rocks, the large rock and the rocks beneath it are firmly connected. Which statement About the forces acting on the balancing rock best explains the stables Bility of the rock overtime Which statement about the forces acting on the balancing rock best explains the stability of the rock over time? Aboutthe forces acting on the balancing rock best explains the stability of the rock over time?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, daryondaniels28
What is the maximum amount of al2(so4)3 which could be formed from 15.84 g of al and 12.89 g of cuso4?
Answers: 2



Chemistry, 22.06.2019 08:00, kleathers97
Nconcentration refers to the molar concentration of an ion in solution. it may be identical to, or greater or less than, the molar concentration of the compound containing the ion that was used to make the solution. for soluble salts, the molarity of a particular ion is equal to the molarity of that compound times the subscript for that ion. for example, 1 m of alcl3 is 1 m in al3+ and 3 m in cl−. 1 m of (nh4)2so4 is 2 m in nh4+ and 1 m in so42−. part a what is the concentration of k+ in 0.15 m of k2s? view available hint(s) nothing m m part b if cacl2 is dissolved in water, what can be said about the concentration of the ca2+ ion? view available hint(s) if is dissolved in water, what can be said about the concentration of the ion? it has the same concentration as the cl− ion. its concentration is half that of the cl− ion. its concentration is twice that of the cl− ion. its concentration is one-third that of the cl− ion. part c a scientist wants to make a solution of tribasic sodium phosphate, na3po4, for a laboratory experiment. how many grams of na3po4 will be needed to produce 550 ml of a solution that has a concentration of na+ ions of 0.700 m ? express your answer numerically in grams. view available hint(s) mass of na3po4 n a 3 p o 4 = nothing g provide feedback
Answers: 3
Do you know the correct answer?
A student is investigating how natural rock formations known as balancing rocks occur. These rock fo...
Questions in other subjects:

Health, 04.11.2020 14:30

English, 04.11.2020 14:30

English, 04.11.2020 14:30


Mathematics, 04.11.2020 14:30

Mathematics, 04.11.2020 14:30

Social Studies, 04.11.2020 14:30

Chemistry, 04.11.2020 14:30

Mathematics, 04.11.2020 14:30







