Answers: 2
Other questions on the subject: Chemistry


Chemistry, 23.06.2019 06:00, girlwonder326
+= + + balance the equation on coefficients, ex. 1,1,1
Answers: 2

Do you know the correct answer?
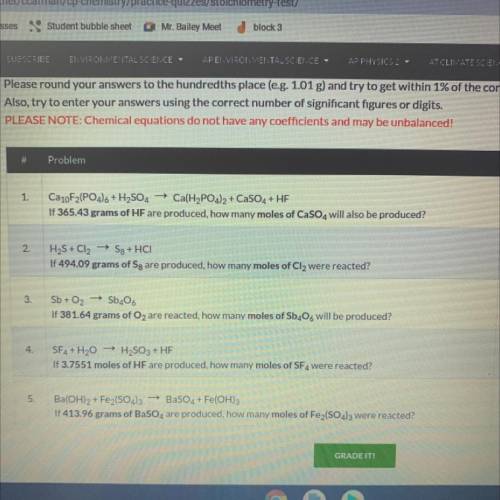
Ca10F2(PO4)6 + H2SO4 + Ca(H2PO4)2 + CaSO4 + HF
If 365.43 grams of HF are produced, how many moles o...
Questions in other subjects:


History, 05.10.2019 00:30

Mathematics, 05.10.2019 00:30




Mathematics, 05.10.2019 00:30



History, 05.10.2019 00:30