
An 18.0 g sample of unidentified metal was heated from 21.5°C to 89.0°C.
292 J of heat energy was absorbed by the metal in the heating process.
Calculate the specific heat of the metal to determine its identity.(need work show, or how you got the answer)
A. calcium
B. iron
C. copper
D. silver
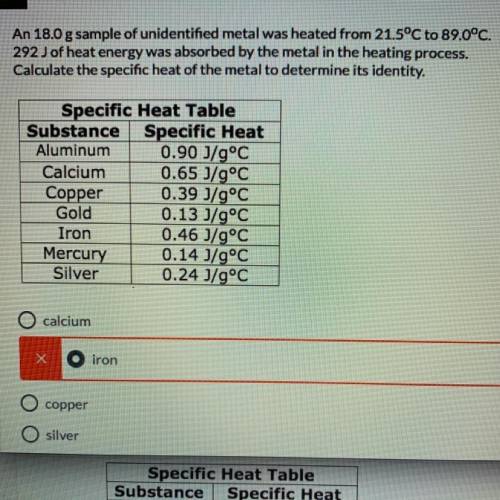

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 23.06.2019 11:30, melissalopez12
Place the following substances in order of ph from lowest ph to highest. a. neutral compounds, bases, acids b. acids, neutral compounds, bases c. bases, acids, neutral compounds d. bases, neutral compounds, acids
Answers: 1

Chemistry, 23.06.2019 12:30, lindseylewis313
When utilizing a transmission electron microscope, why is it necessary to stain the specimen with heavy metal salts?
Answers: 1

Chemistry, 23.06.2019 16:10, reesemf2006
What type of reaction is shown below? check all that apply. agno3(aq) + nacl(aq) → nano3(aq) + agcl(s) i synthesis decomposition combustion i single replacement double replacement done
Answers: 2
Do you know the correct answer?
An 18.0 g sample of unidentified metal was heated from 21.5°C to 89.0°C.
292 J of heat energy was a...
Questions in other subjects:






Social Studies, 19.09.2019 05:30


Mathematics, 19.09.2019 05:30


Mathematics, 19.09.2019 05:30


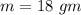
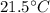 to
to 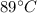
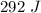
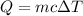
![\Rightarrow 292=18\times c\times [89-21.5]\\\Rightarrow 292=18\times c\times 67.5\\\\\Rightarrow c=\dfrac{292}{1215}\\\\\Rightarrow c=0.24\ J/g^{\circ}C](/tpl/images/1318/5230/23712.png)




