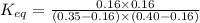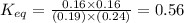In the coal-gasification process, carbon monoxide is converted to carbon dioxide via the following reaction: CO (g) H2O (g) CO2 (g) H2 (g) In an experiment, 0.35 mol of CO and 0.40 mol of H2O were placed in a 1.00-L reaction vessel. At equilibrium, there were 0.19 mol of CO remaining. Keq at the temperature of the experiment is . A) 5.47 B) 1.0 C) 1.78 D) 0.75 E) 0.56

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:40, cheesecake1919
Which diagram shows the correct way to represent an ionic compound of magnesium oxide?
Answers: 3



Chemistry, 23.06.2019 01:00, bsheepicornozj0gc
Reactions in cells take place at about a. 40°c b. 0° c. 100°c d. 60°c
Answers: 1
Do you know the correct answer?
In the coal-gasification process, carbon monoxide is converted to carbon dioxide via the following r...
Questions in other subjects:




English, 04.10.2019 23:30


Mathematics, 04.10.2019 23:30

History, 04.10.2019 23:30


Mathematics, 04.10.2019 23:30

Mathematics, 04.10.2019 23:30


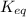 at the temperature of the experiment is 0.56.
at the temperature of the experiment is 0.56.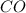 = 0.35 mole
= 0.35 mole
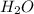 = 0.40 mole
= 0.40 mole
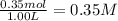
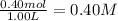
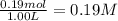
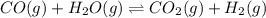
![K_{eq}=\frac{[CO_2]\times [H_2]}{[CO]\times [H_2O]}](/tpl/images/1317/1212/44bdf.png)