
Chemistry, 11.05.2021 21:20, fhbuvgy7836
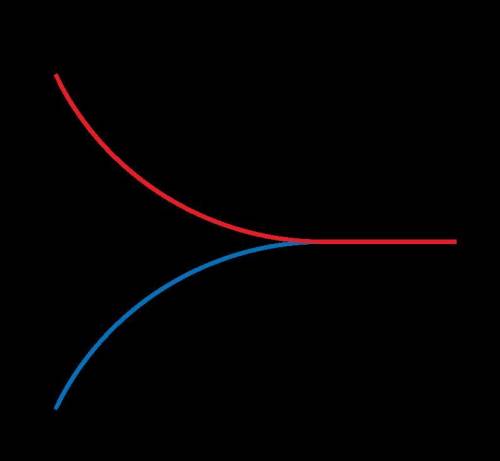
What is true in this reaction?
The reaction will go to completion because the rate of the forward reaction is greater than the rate of the reverse reaction.
The reaction does not reach equilibrium because the rates of the forward and reverse reactions are different.
The reaction reaches chemical equilibrium when the rates of the forward and reverse reactions are equal.
Whether the reaction is at equilibrium cannot be determined by looking at the graph.

Answers: 3
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 14:30, CoolRahim9090
Which of the following represents the ester functional group? a. -coo- b. -cho c. -cooh d. c=o
Answers: 1

Chemistry, 22.06.2019 15:00, tcapele252
‘which reaction would most likely require the use of an inert electrode?
Answers: 1
Do you know the correct answer?
What is true in this reaction?
The reaction will go to completion because the rate of the forward r...
Questions in other subjects:

Mathematics, 29.08.2019 07:10

Social Studies, 29.08.2019 07:10


Mathematics, 29.08.2019 07:10

Mathematics, 29.08.2019 07:10


Mathematics, 29.08.2019 07:10

Biology, 29.08.2019 07:10

Chemistry, 29.08.2019 07:10






