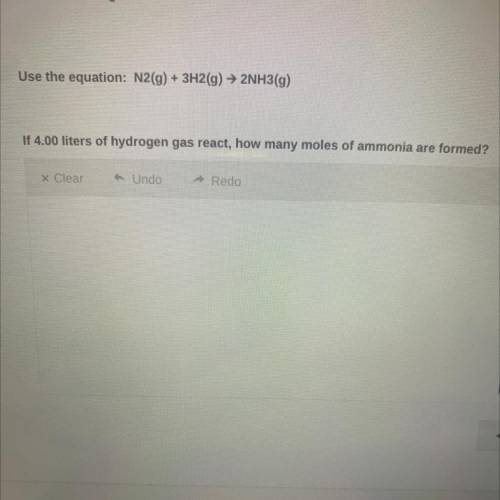
Use the equation:
N2(g) + 3H2(g) → 2NH3(g)
If 4.00 liters of hydrogen gas react, how ma...


Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 07:30, nayiiii1874
What three things determine the shape and size of a puddle when water is poured out onto a surface
Answers: 2

Chemistry, 22.06.2019 21:30, Turtlelover05
How can the periodic table be used to predict the behavior of elements?
Answers: 1

Chemistry, 23.06.2019 00:30, mathwiznot45
Element j is 1s 2s 2p 3s . (i) how many unpaired electrons does j have? (ii) is j a good oxidizing agent or a reducing agent? (iii) state reason for the answer.
Answers: 1
Do you know the correct answer?
Questions in other subjects:


Mathematics, 18.04.2021 16:10



Mathematics, 18.04.2021 16:10





Physics, 18.04.2021 16:10