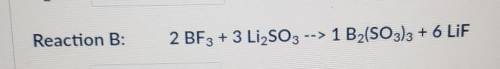Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 06:40, alyons60
Which statement correctly describes metallic bonds? a. they form when certain atoms lose electrons and other atoms gain electrons. b. they involve an attraction between anions and cations. they always involvpoth a metal and a nonmetal. d. they can only form between atoms of the same element. e. they form because electrons can move freely between atoms.
Answers: 3

Chemistry, 22.06.2019 12:30, nekathadon
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1

Chemistry, 22.06.2019 12:30, meghan2529
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1
Do you know the correct answer?
According to Reaction B, how many moles of B2(SO3)3 can be formed from 9.31 moles of LiF?
3.10 mol...
Questions in other subjects:


Mathematics, 24.03.2021 20:00



English, 24.03.2021 20:00


History, 24.03.2021 20:00

English, 24.03.2021 20:00