
Chemistry, 10.05.2021 14:00, amiechap12
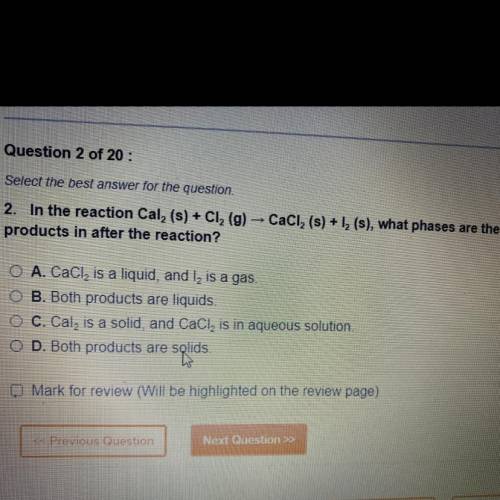
In the reaction Cal 2 (s)+Cl 2 (g) CaCl 2 (s)+l 2 (s) , what phases are the products in after the reaction ?
A. CaCl 2 is a liquid, and l2 is a gas
B. Both products are a liquid
C. Cal2 is a solid, CaCl2 is in aqueous solution
D. Both products are solids

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 11:20, ashiteru123
Which of the following contributes to the structural rigidity of cellulose? adjacent glucose polymers are stabilized by hydrogen bonding. glucose residues are joined by (α1→4) linkages. cellulose is a highly branched molecule. the conformation of the glucose polymer is a coiled structure.
Answers: 2

Chemistry, 22.06.2019 13:30, citlalli30
1) which of the following is the best example of a physical change? a) sugar dissolving in tea b) firefly glowing 2) in the combustion of ethane, what is/are the reactants? c2h6 + o2 ==> co2 + h2o a) c2h6 and o2 b) co2 and c2h6
Answers: 2

Chemistry, 22.06.2019 15:20, shanyeah
Water is initially present in a state where its molecules are far apart. during a change of state, its molecules slow down. which change of state has most likely taken place? from a gas to a liquid from a liquid to a gas from a solid to a liquid from a gas to a plasma
Answers: 1
Do you know the correct answer?
In the reaction Cal 2 (s)+Cl 2 (g) CaCl 2 (s)+l 2 (s) , what phases are the products in after the re...
Questions in other subjects:

Mathematics, 04.11.2020 17:20



Arts, 04.11.2020 17:20

English, 04.11.2020 17:20

Business, 04.11.2020 17:20

Mathematics, 04.11.2020 17:20

History, 04.11.2020 17:20


Mathematics, 04.11.2020 17:20






