
Chemistry, 08.05.2021 22:50, lerasteidl
How many moles of carbon dioxide are produced in the reaction between hydrochloric acid and calcium carbonate when 2 moles of HCl are used to start with?
2HCl + CaCO3 +CaCl2 + CO2 + H2O
A. 2
B. 4
C. 1
D. 3
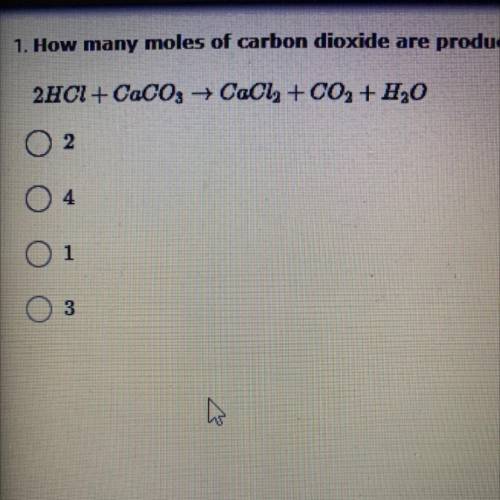

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, palcochran1313
Describe the interaction that occurs between two objects with the same electrical charge.
Answers: 1

Chemistry, 22.06.2019 17:10, mikeeway33
Some liquids can be distilled, but only at temperatures that are so high that it is impractical, or so high the compound decomposes. explain why distillation such compounds at significantly less than atmospheric pressure (some degree of vacuum) would solve this problem.
Answers: 2

Chemistry, 22.06.2019 23:00, poolwaterisgross
How does the value of the equilibrium constant show that a reaction reaches equilibrium very quickly? (a) the equilibrium constant is large. (b) the equilibrium constant is small. (c) the equilibrium constant is zero. (d) the value of the equilibrium constant does not show how quickly a reaction comes to equilibrium.
Answers: 1
Do you know the correct answer?
How many moles of carbon dioxide are produced in the reaction between hydrochloric acid and calcium...
Questions in other subjects:

Mathematics, 23.06.2019 03:40


Mathematics, 23.06.2019 03:40


Mathematics, 23.06.2019 03:40

Mathematics, 23.06.2019 03:40



Mathematics, 23.06.2019 03:40

Arts, 23.06.2019 03:40






