
Chemistry, 08.05.2021 08:10, sparky1234
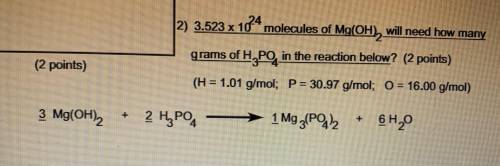
3.523 x 10^24 molecules of Mg(OH2) will need how many grams of H3PO4 in the reaction below
H=1.01 g/mol
P=30.97 g/mol
O=16.00 g/mol
3 Mg(OH)2 + 2 H3PO4 -> 1 Mg3 (PO4 )2 + 6 H2O

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, aubreykenzie686
Naoki's bicycle has a mass of 10 kg. if naoki sits on her bicycle and starts pedaling with a force of 168 n, causing an acceleration of 2.8 m/s2, what is naoki's mass?
Answers: 1

Chemistry, 22.06.2019 18:00, jalenclarke25
What volume would 2.25 moles of ne has occupy at stp?
Answers: 1

Chemistry, 22.06.2019 22:30, teagan56
Gusing the milligrams of ascorbic acid you entered above, the ratio of total sample volume to aliquot volume, and the total milligrams of the vitamin c tablet that you dissolved, calculate the mass of ascorbic acid in the vitamin c tablet for each trial. do this by scaling up to find the amount (mg) of ascorbic acid in your 250 ml flask. enter your calculated mass of ascorbic acid in the vitamin c tablet, for each trial. be sure to enter your calculated mass in the corresponding order that you entered your milligrams of ascorbic acid. the milligrams of ascorbic acid you entered for entry #1 previously should correspond to the mass of ascorbic acid that you enter for entry #1 here.
Answers: 1

Chemistry, 23.06.2019 00:30, evelynalper08
Which radioisotope is used to date fossils? a. oxygen-16 b. carbon-14 c. uranium-238 d. carbon-12
Answers: 2
Do you know the correct answer?
3.523 x 10^24 molecules of Mg(OH2) will need how many grams of H3PO4 in the reaction below
H=1.01...
Questions in other subjects:



Mathematics, 11.03.2020 02:39



Mathematics, 11.03.2020 02:39


Mathematics, 11.03.2020 02:39


Mathematics, 11.03.2020 02:39






