
Chemistry, 07.05.2021 22:30, aallyssabrown0120
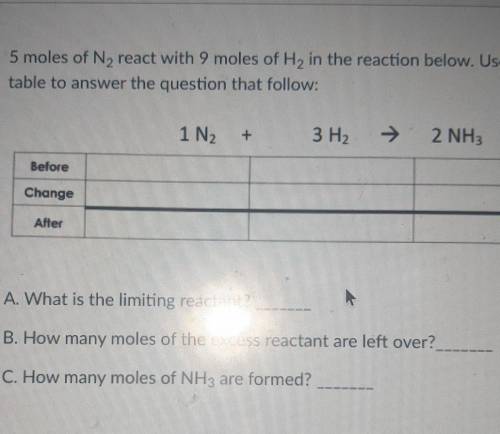
5 moles of N2 react with 9 moles of H2 in the reaction below. Use the BCA table to answer the question that follow: 1N2+3H2=2NH3
A. What is the limiting reactant?
B. How many moles of the excess reactant are left over?
C. How many moles of NH3 are formed?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:50, aletadaboss
Acompound contains c, h, and o atoms. when 1.130 g of the compound is burned in oxygen, 1.064 g co2 and 0.3631 g h2o are produced. what is the empirical formula of this compound?
Answers: 1


Chemistry, 22.06.2019 12:00, angtrevv
In a laboratory, 1.55mg of an organic compound containing carbon, hydrogen, and oxygen is burned for analysis. this combustion resulted in the formation of 1.45mg of carbon dioxide and .89 mg of water. what is the empirical formula for this compound?
Answers: 1

Chemistry, 22.06.2019 18:00, AdoNice
Many pharmaceutical drugs are organic compounds that were originally synthesized in the laboratory. which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 2
Do you know the correct answer?
5 moles of N2 react with 9 moles of H2 in the reaction below. Use the BCA table to answer the questi...
Questions in other subjects:

Mathematics, 14.09.2020 22:01

Mathematics, 14.09.2020 22:01

Social Studies, 14.09.2020 22:01

Mathematics, 14.09.2020 22:01

Social Studies, 14.09.2020 22:01

Mathematics, 14.09.2020 22:01

Mathematics, 14.09.2020 22:01

Mathematics, 14.09.2020 22:01

Physics, 14.09.2020 22:01

Mathematics, 14.09.2020 22:01






