
Chemistry, 07.05.2021 22:20, myamiller558
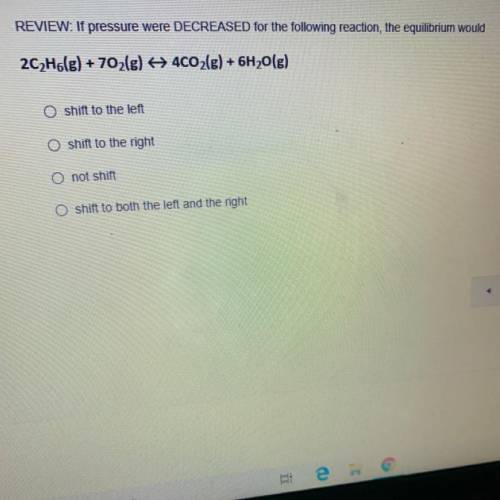
REVIEW: If pressure were DECREASED for the following reaction, the equilibrium would 2C2H6(g) + 702(g) + 4CO2(g) + 6H2O(g)

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:00, nauticatyson9
Jose and eric were given four samples in lab. the results of their analysis are shown in the table. based on the data they collected, which sample is most likely a metal?
Answers: 1

Chemistry, 22.06.2019 15:30, dylannhandy
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 2


Chemistry, 22.06.2019 21:30, kawaiiblurainbow
Which of the following changes will decrease the total amount of gaseous solute able to be dissolved in a liter of liquid water? (2 points) decreasing temperature decreasing pressure decreasing surface area decreasing solute concentration
Answers: 1
Do you know the correct answer?
REVIEW: If pressure were DECREASED for the following reaction, the equilibrium would
2C2H6(g) + 70...
Questions in other subjects:

Social Studies, 14.10.2019 18:10

Mathematics, 14.10.2019 18:10













