
Chemistry, 07.05.2021 20:50, whiteshawn0250
A 3.00 L Flask contains 1.60 moles of a gas at 27.0°C. Choose the correct equation to solve for the pressure of the gas in mmHg?
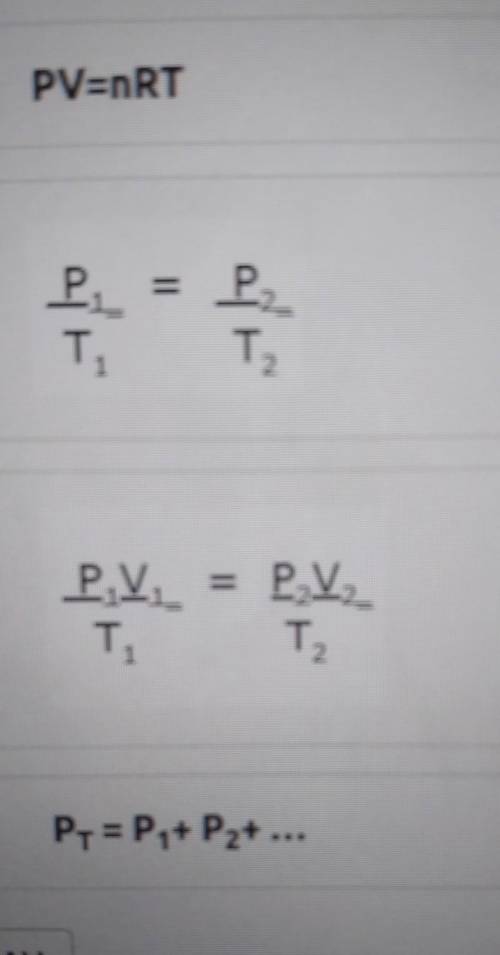

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 23.06.2019 00:50, trinityine
50 points! need answer asap. what type of organic compound contains the following functional group? (2 points)
Answers: 3

Chemistry, 23.06.2019 01:30, AptAlbatross
Use the periodic table to determine how many grams of oxygen would be required to react completely with 859.0 g c2h2
Answers: 3

Chemistry, 23.06.2019 01:50, kayleebueno
Drag the tiles to the correct locations. each tile can be used more than once, but not all tiles will be used. one or more locations will remain empty. nitrosyl fluoride has the chemical formula nof nitrogen has five valence electrons, oxygen has six, and fluorine has seven. complete the lewis structure for this covalent compound. f n = = = . : : 0 : reset next um. all rights reserved us 2
Answers: 2
Do you know the correct answer?
A 3.00 L Flask contains 1.60 moles of a gas at 27.0°C. Choose the correct equation to solve for the...
Questions in other subjects:
















