
1. Calculation of equilibrium concentrations from Ka
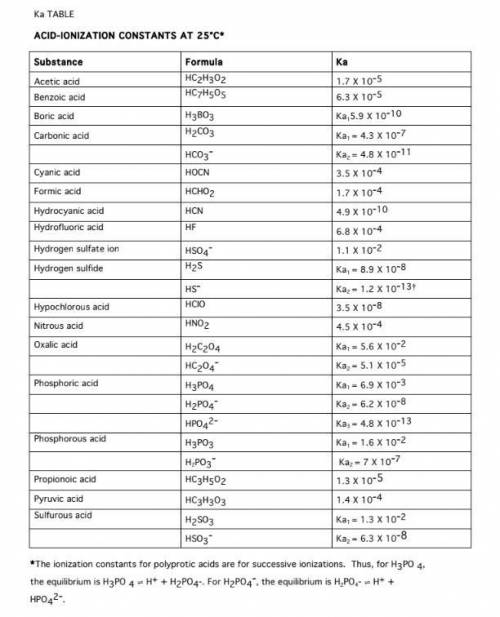
Calculate the pH of a 1.0 M Acetic acid solution, using approximations. Ka = 1.8 x 10^-5
HC2H3O2+H2O⇆H3O(+)+C2H3O2(-)
Initial:
Change
at Equilibrium:
2. Calculation of species concentrations from Ka, using the quadratic formula
Calculate the pH of a 0.000010 M Acetic acid solution
HC2H3O2+H2O⇆H3O(+)+C2H3O2(-)
Initial:
Change:
at Equilibrium:
3. Calculation of Ka from the pH of a weak acid solution
Calculate the Ka of HNO2 if a 0.10 M HNO2 solution has a pH of 2.187
Initial:
Change:
at Equilibrium:
4. Calculation of Ka from the percent ionization
Calculate the Ka of Glycine if a 0.10 M Glycine solution is 4.1 x 10^-3 ionized
HGly+H2O⇆H3O(+)+Gly(-)
Initial:
Change:
at Equilibrium:

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 05:30, mandy9386
You are making a solution of calcium chloride dissolved in water. you add solid, stir, and it dissolves. you add just a spatula tip full, stir, and the solid does not dissolve. how could you describe the solutions before and after adding the spatula tip amount
Answers: 1


Chemistry, 22.06.2019 14:30, villarrealc1987
In water, a strong acid will break down into its component parts. a. completely b. partly c. never in water, a weak base will break down into its component parts. a. completely b. partly c. never
Answers: 2
Do you know the correct answer?
1. Calculation of equilibrium concentrations from Ka
Calculate the pH of a 1.0 M Acetic acid solut...
Questions in other subjects:

Business, 30.01.2021 01:00

Mathematics, 30.01.2021 01:00

Chemistry, 30.01.2021 01:00


Mathematics, 30.01.2021 01:00

English, 30.01.2021 01:00

Mathematics, 30.01.2021 01:00

Chemistry, 30.01.2021 01:00

Mathematics, 30.01.2021 01:00

Mathematics, 30.01.2021 01:00






