
Chemistry, 07.05.2021 04:10, brookedeanovich
NO LINKS I WILL REPORT U
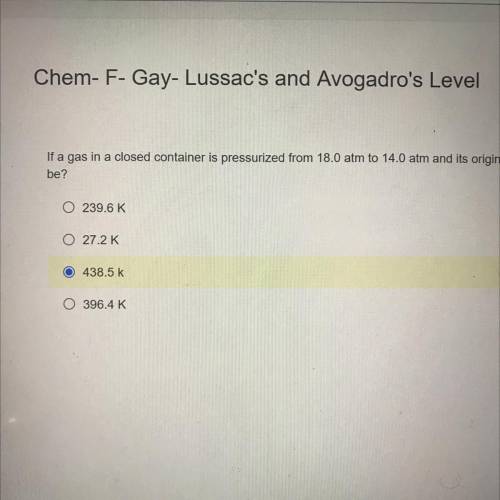
If a gas in a closed container is pressurized from 18.0 atm to 14.0 atm and its original temperature was 35.0°C, what would the final temperature of the gas
be?
239.6K
27.2K
. 438.5 K
396.4 K

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:30, davidrodriguez122001
Which of the following describes a situation where competition between producers exists
Answers: 1

Chemistry, 22.06.2019 16:00, yfnal3x
What rule is used to determine how many covalent bonds an element can form? a. the number of covalent bonds is equal to six c the number of covalent bonds is equal to five minus the group number plus the group number b. the number of covalent bonds is equal to eight d. none of the above minus the group number select the best answer from the choices provided
Answers: 2

Chemistry, 22.06.2019 18:00, LuvieAnn1886
How is energy related to the change of state represented by the model? atoms gain energy as a solid changes to a liquid. atoms gain energy as a solid changes to a gas. atoms lose energy as a solid changes to a liquid. atoms lose energy as a solid changes to a gas.
Answers: 3
Do you know the correct answer?
NO LINKS I WILL REPORT U
If a gas in a closed container is pressurized from 18.0 atm to 14.0 atm a...
Questions in other subjects:


History, 04.08.2020 14:01




Mathematics, 04.08.2020 14:01









