
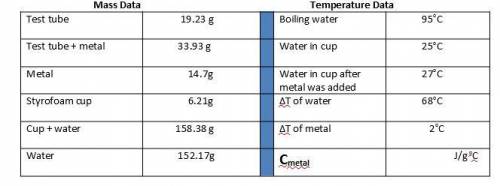
1. Calculate the heat gained by the water (lost by the metal) in the calorimeter using the equation in the introduction.
Metal A
Q = mc(ΔT)
Qwater = -Qmetal
Heat gained = Mass of x Specific heat of x Change in temperature
by the water water (g) water (4.184 J/goC) (ΔT)
The specific heat of the metal can now be calculated:
Specific heat = Heat gained by the water
of metal (c) Mass of metal (g) x ΔT of metal (oC)

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:20, JotaroKujo6233
Determine which intermolecular forces are the dominant (strongest) forces for a pure sample of each of the following molecules by placing the molecules into the correct bins. drag the appropriate molecular formula to their respective bins.
Answers: 3


Chemistry, 22.06.2019 08:00, tchase0616
What is the molarity of 60.0 grams of naoh dissolved in 750 milliliters of water? a) 1.1 m b) 2.0 m c) 12 m d) 75 m
Answers: 1

Chemistry, 22.06.2019 17:30, tiffanyhmptn
Which scenario is most similar to the type of collision that gas particles have according to kinetic molecular theory
Answers: 1
Do you know the correct answer?
1. Calculate the heat gained by the water (lost by the metal) in the calorimeter using the equation...
Questions in other subjects:


Geography, 23.10.2021 21:00

History, 23.10.2021 21:00



Biology, 23.10.2021 21:00

English, 23.10.2021 21:00

Mathematics, 23.10.2021 21:00







