70.8 g of CO is exposed 20.4 g of Hy and a reaction takes place.
CO + 2H2 - CH3OH
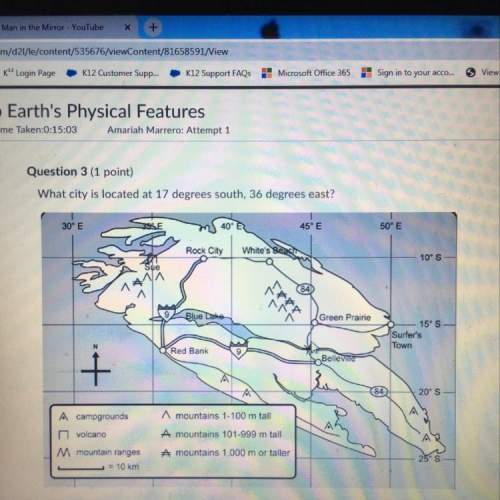
1. How man...

70.8 g of CO is exposed 20.4 g of Hy and a reaction takes place.
CO + 2H2 - CH3OH
1. How many grams of methyl hydroxide (CH3OH) is produced?
2. What is the limiting reactant?
3. How much of the excess reactant is left unused?
literally what does any of this mean

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:40, tatemelliott
Calculate the number of kilojoules to warm 125 g of iron from 23.5°c to 78.0°c.
Answers: 3


Do you know the correct answer?
Questions in other subjects:


Mathematics, 30.01.2021 19:40

English, 30.01.2021 19:40

Mathematics, 30.01.2021 19:40

Mathematics, 30.01.2021 19:40





Biology, 30.01.2021 19:40