
Chemistry, 06.05.2021 23:40, Thiskid100
There are 3.6 moles of Mg and 5.1 moles of O2 what is the limiting reactant the excess reactant and the theoretical yield?
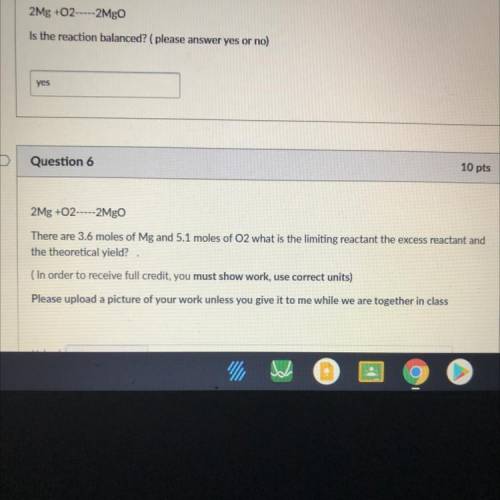

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:10, amuijakobp78deg
Agas mixture with a total pressure of 745 mmhg contains each of the following gases at the indicated partial pressures: co2, 245 mmhg ; ar, 119 mmhg ; and o2, 163 mmhg . the mixture also contains helium gas. part a what is the partial pressure of the helium gas? phe p h e = nothing mmhg request answer part b what mass of helium gas is present in a 10.2-l sample of this mixture at 283 k ? m m = nothing g request answer
Answers: 1



Chemistry, 23.06.2019 00:50, maddysmall32
Which of the following warnings would an agricultural chemist tell a farmer who wants to recycle his or her own ammonia? recycling ammonia is a difficult process that sometimes takes weeks. recycling ammonia requires a degree in biochemistry or a related field. recycling ammonia can be harmful because it is highly flammable and toxic. recycling ammonia costs too much money considering the price of the necessary chemicals.
Answers: 1
Do you know the correct answer?
There are 3.6 moles of Mg and 5.1 moles of O2 what is the limiting reactant the excess reactant and...
Questions in other subjects:

Mathematics, 27.10.2020 19:30

Mathematics, 27.10.2020 19:30

Social Studies, 27.10.2020 19:30



Biology, 27.10.2020 19:30

Mathematics, 27.10.2020 19:30

English, 27.10.2020 19:30

Mathematics, 27.10.2020 19:30

Geography, 27.10.2020 19:30






