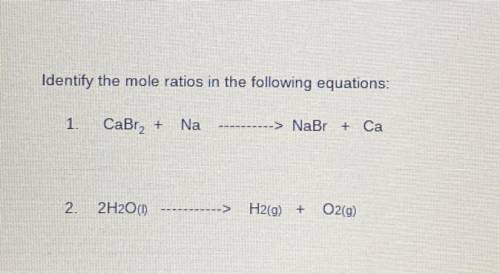
Identify the mole ratios in the following equations:
1. CaBra2 + Na= NaBr + Ca
2. 2H20 (I)=H...

Chemistry, 06.05.2021 21:00, wolfgirl4762
Identify the mole ratios in the following equations:
1. CaBra2 + Na= NaBr + Ca
2. 2H20 (I)=H2(g) + O2(9)

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:00, hjamya17
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 1

Chemistry, 22.06.2019 11:00, jodonw5616
The diagram below shows the different phase transitions that occur in matter. which arrow represents the transition in which dew is formed?
Answers: 1

Do you know the correct answer?
Questions in other subjects:

History, 15.11.2020 02:40

Chemistry, 15.11.2020 02:40


Mathematics, 15.11.2020 02:40

Mathematics, 15.11.2020 02:40

English, 15.11.2020 02:40


History, 15.11.2020 02:40

English, 15.11.2020 02:40

Mathematics, 15.11.2020 02:40






