

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:30, Cartucho1978
According to le chatelier’s principle, a system in chemical equilibrium responds to stress by shifting the equilibrium in a direction that reduces the stress. normalizes the stress. increases the stress. changes the stress.
Answers: 1

Chemistry, 22.06.2019 20:00, montimcdaniel
If one fission reaction of a uranium-235 atom produced two neutrons, how many neutrons would be released if the chain reaction occurred three more times?
Answers: 1

Chemistry, 22.06.2019 23:30, johnnysteeler9934
The ammonia molecule in the diagram has the observed bond orientation because
Answers: 1

Chemistry, 23.06.2019 04:00, ayoismeisjjjjuan
How many liters of water can be produced from 5.0liters of butane gas at stp, assuming excess oxygen? c4h10(g) + 02(g) → co2 (e) + h2o (g)
Answers: 2
Do you know the correct answer?
4. Balance the following equation. Then determine the number of mols of Nitrogen formed given the va...
Questions in other subjects:

Social Studies, 21.01.2021 19:10


Mathematics, 21.01.2021 19:10




Biology, 21.01.2021 19:10

English, 21.01.2021 19:10

Mathematics, 21.01.2021 19:10

Mathematics, 21.01.2021 19:10


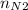 = 4 × 2 ÷ 4 = 2
= 4 × 2 ÷ 4 = 2 



