
Chemistry, 05.05.2021 08:40, EllaLovesAnime
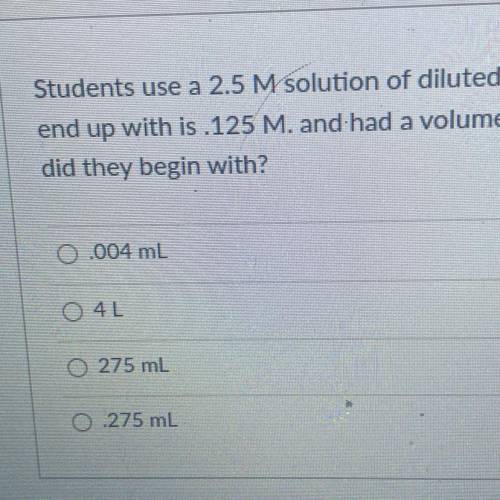
Students use a 2.5 M solution of diluted hydrochloric acid. The Molarity of the solution they
end up with is 125 M. and had a volume of 5500mL. What volume of the concentrated HCI
did they begin with?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:20, sarinaneedshelp01
Match the acid base pairs by arranging the acid name with the conjugate base formula. hydrogen carbonate hydrogen phosphate carbonic acid read water sulfuric acid phosphoric acid a. co32- b. hso4- c. hco3- d. po43- e. h2po4- f. oh-
Answers: 1

Chemistry, 22.06.2019 05:30, sethjohnson386pbnm3x
Modern weaponry has increased the number of deaths in wars and violent conflicts.
Answers: 3

Chemistry, 22.06.2019 06:10, andybiersack154
Explain the relationship between forward and backward reactions in equilibrium, and predict how changing the amount of a reactant (creating a tension) will affect that relationship.
Answers: 1

Chemistry, 22.06.2019 13:00, yaneiryx5476
Is 9 correct? and can someone me with 10? it’s due tomorrow, you
Answers: 1
Do you know the correct answer?
Students use a 2.5 M solution of diluted hydrochloric acid. The Molarity of the solution they
end...
Questions in other subjects:



Mathematics, 17.12.2020 17:00



Physics, 17.12.2020 17:00


English, 17.12.2020 17:00

Physics, 17.12.2020 17:00






