
Chemistry, 05.05.2021 01:00, caliharris123
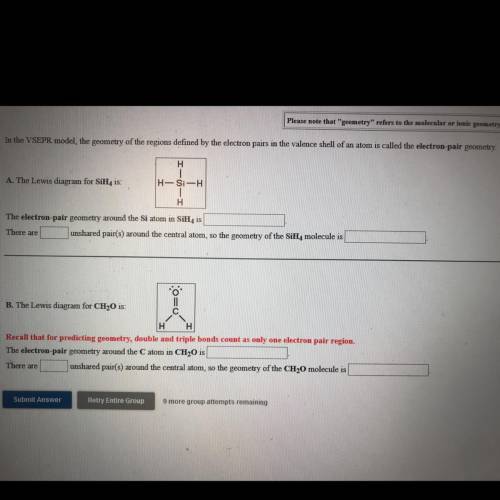
In the VSEPR model, the geometry of the regions defined by the electron pairs in the valence shell of an atom is called the electron-pair geometry.
H
A. The Lewis diagram for SiH, is:
H-Si-H
1
H
The electron pair geometry around the Si atom in SiH, is
There are unshared pair(s) around the central atom, so the geometry of the SiH, molecule is
.
11
B. The Lewis diagram for CH20 is:
C с
H H
Recall that for predicting geometry, double and triple bonds count as only one electron pair region.
The electron pair geometry around the Catom in CH20 is
There are unshared pair(s) around the central atom, so the geometry of the CH2O molecule is

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:00, advancedgamin8458
Consider the nuclear equation below. 239 > x + 4 he 94 2 what is x? 1.235 cm 96 2.243 u 92 3.235 u 92 4.243 cm 96
Answers: 2



Chemistry, 22.06.2019 16:50, struckedblazing
Answer asap need it by wednesday morning calculate the ph of 0.02m hcl best answer will be brainliest
Answers: 1
Do you know the correct answer?
In the VSEPR model, the geometry of the regions defined by the electron pairs in the valence shell o...
Questions in other subjects:















