
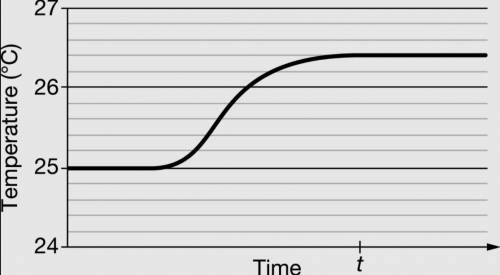
A student did an experiment to determine the specific heat capacity of a metal alloy. The student put a sample of the alloy in boiling water for several minutes, then quickly transferred the alloy into a calorimeter containing water originally at 25°C. The temperature of the water was monitored over time. The data are given in the graph above.
(a) What is the value of ΔT that the student should use to calculate the value of q, the heat gained by the water?
(b) In terms of what occurs at the particulate level, explain how the temperature of the water increases after the alloy sample is added.
(c) The student claims that thermal equilibrium is reached at time t. Justify the student’s claim. In your justification, include a description of what occurs at the particulate level when the alloy and the water have reached thermal equilibrium.

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 06:00, Kjswagout5052
In an investigation that uses the scientific method, which step immediately follows making a hypothesis? o summarizing the results o asking a question o making observations designing an experiment mark this and retum save and exit next submit
Answers: 2

Chemistry, 22.06.2019 06:30, angelrenee2000
Ineed someone to see if my answers are correct! if any are wrong let me know what the correct answers would be and how to get that answer! 1. how many moles of sodium chloride are in 28 grams od nacl? a. 265 mole naclb. 856 mole naclc. 479 mole of nacld. 1.2 mole nacl < my choice2. 734 grams of lithium sulfate (li2so4) are dissolved to make 2500 ml of solution what is rhe molaratiy? a. 2.67 mb. 4.56 mc. 3.89 m < my choiced. 1.78 m3. how many grams of cacl2 would be dissolved in 3.0 l of a 0.50 m solution of cacl2? a. 250 g cacl2 b. 166.5 g cacl2c. 113.65 g cacl2d. 98 g cacl2 < my choice4. suppose you had 58.44 g of nacl and you dissolved it in exactly 2.00 liters. the molarity if the solution would be 0.5 mtrue < my choicefalse 5. i would need 22g of naoh to make a 3.0 m solution using 250 ml of solvent. true < my choicefalse6. identify the solute: you have a .0195 m solution made from using 6.5 g of solute and 3 l of solvent. identify the solute by solving for molar weight. a. the solute is nacl because the molar weight is 58.43 g/mol < my choiceb. the solute is h2so4 because the molar weight is 98.06 g/molc. the solute is cacl2 because the molar weight is 111.11 g/mol
Answers: 1
Do you know the correct answer?
A student did an experiment to determine the specific heat capacity of a metal alloy. The student pu...
Questions in other subjects:

Mathematics, 06.12.2019 06:31


Mathematics, 06.12.2019 06:31


History, 06.12.2019 06:31











