
Chemistry, 03.05.2021 21:10, estefaniapenalo
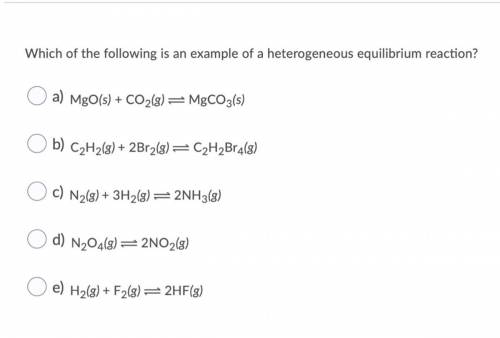
Which of the following is an example of a heterogeneous equilibrium reaction? O a) MgO(s) + CO2(g) = MgCO3(s) O b) C2H2(g) + 2Br2(g) = C2H2Br4(8) O c) N2(g) + 3H2(g) = 2NH3(8) O d) N204(8) =2NO2(8) O e) H2(g) + F2(8)=2HF(3)

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 03:00, ian2006huang
Which of these would be caused by a chemical change? a) the formation of lava. b) sedimantary rock layering over time. c) metamorphic rock forming from igneous. d) metamorphic rock eroding to form sedimentary rock.
Answers: 3

Chemistry, 22.06.2019 05:00, YoEsMyles3115
0.2348 grams of pbcl2 used to form 44.0 ml of solution.
Answers: 1

Chemistry, 23.06.2019 01:00, akluke6059
Which statement characterizes synthetic polymers? a. they come from animals and plants. b. they are found in nature. c. they are made in a lab. d. they are components of starch.
Answers: 1
Do you know the correct answer?
Which of the following is an example of a heterogeneous equilibrium reaction? O a) MgO(s) + CO2(g) =...
Questions in other subjects:

Mathematics, 05.10.2020 21:01

Mathematics, 05.10.2020 21:01

Physics, 05.10.2020 21:01




Mathematics, 05.10.2020 21:01

Mathematics, 05.10.2020 21:01

History, 05.10.2020 21:01







