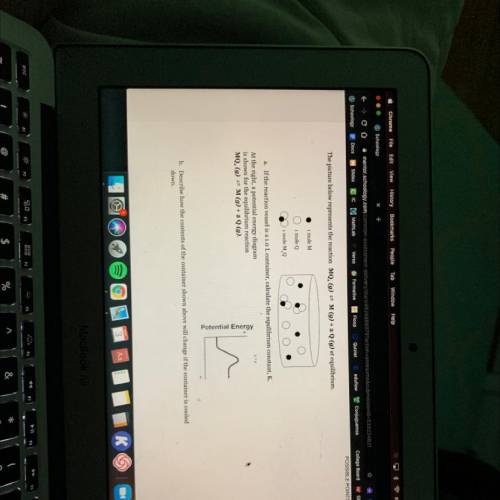
The picture below represents the reaction MQ, (g) = M(g) + 2 Q (g) at equilibrium.
1 mole M
...

Chemistry, 03.05.2021 20:10, hihihi129473838
The picture below represents the reaction MQ, (g) = M(g) + 2 Q (g) at equilibrium.
1 mole M
i mole Q
1 mole MQ
a. If the reaction vessel is a 1.0 L container, calculate the equilibrium constant, K.
At the right, a potential energy diagram
is shown for the equilibrium reaction
MQ. (g) = M(g) + 2 Q (9).
Potential Energy
b. Describe how the contents of the container shown above will change if the container is cooled
down.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:30, cynthiagutierrez65
Given that the molar mass of nano3 is 85.00 g/mol, what mass of nano3 is needed to make 4.50 l of a 1.50 m nano3solution? use .6.75 g18.9 g255 g574 g
Answers: 1

Chemistry, 21.06.2019 22:00, NREYESLDS2806
To save time, you can approximate the initial mass of the solid to the nearest ±1 g. for example, if you are asked to add 14.3 g of copper, add between 13 g and 15 g. which of the following sets include two samples with an equal density? which all that apply below 15.4 g gold and 18.7 g silver 15.2 g copper and 50.0 g copper 20.2 g silver and 20.2 g copper 11.2 g gold and 14.9 g gold
Answers: 1

Chemistry, 21.06.2019 22:30, shantrice1831
Using the periodic table, complete the table to describe each atom. type in your answers. a ? b? c? d? e? f?
Answers: 1

Chemistry, 22.06.2019 09:00, tashaunalewis4786
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 1
Do you know the correct answer?
Questions in other subjects:

Mathematics, 28.10.2020 23:30


Mathematics, 28.10.2020 23:30

Mathematics, 28.10.2020 23:30


Social Studies, 28.10.2020 23:30


Mathematics, 28.10.2020 23:30

Mathematics, 28.10.2020 23:30

Mathematics, 28.10.2020 23:30






