
Chemistry, 03.05.2021 19:10, joshua13338
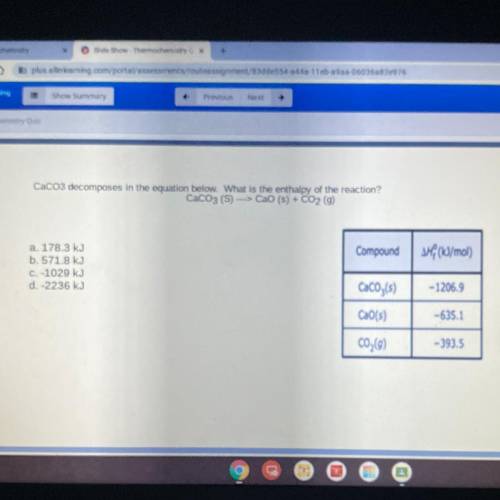
CaCo3 decomposes in the equation below. What is the enthalpy of the reaction?
CaCO3 (S) ---> CaO (s) + CO2 (g)
a. 178.3 kJ
b. 571.8 kJ
C. -1029 kJ
d. -2236 kJ

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 15:00, YoVeoAnime
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 1

Chemistry, 22.06.2019 17:00, emma3216
In a heat engine of 1000 j of heat enters the system and the piston does 500 j of work what is the final internal energy of the system if the inital energy was 2000 j we have to do all of these down here 1)write the equation 2)list out your know variables 3)plug the numbers into the equations 4)solve 5)write your solution statemtn that includes inital energuy and final energuy added
Answers: 1

Chemistry, 22.06.2019 17:10, glitterpanda2468
Calculate the estimated density of each ball. use the formula d = m/v where d is the density, m is the mass, and v is the volume. record your calculations in table a of your student guide. given that the density of water is 1.0 g/cm3, make a prediction about whether each ball will float in water. record your prediction in table a. what is the estimated density of the table tennis ball? record your answer to the nearest hundredth
Answers: 2
Do you know the correct answer?
CaCo3 decomposes in the equation below. What is the enthalpy of the reaction?
CaCO3 (S) ---> Ca...
Questions in other subjects:




Mathematics, 09.09.2021 22:50


Mathematics, 09.09.2021 22:50

Physics, 09.09.2021 22:50


Health, 09.09.2021 22:50






