
Chemistry, 03.05.2021 19:00, kcutler8603
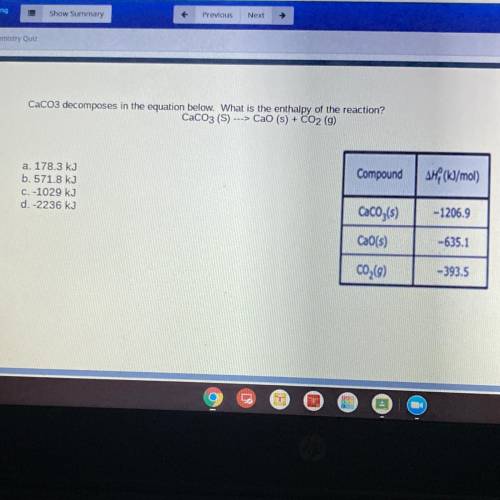
CaCO3 decomposes in the equation below. What is the enthalpy of the reaction?
CaCO3 (S) ---> Cao (s) + CO2 (g)
Compound
AH (kJ/mol)
a. 178.3 kJ
b. 571.8 kJ
C. -1029 kJ
d.-2236 kJ
CaCO3(s)
-1206.9
CaO(s)
-635.1
C02(9)
-393.5

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:00, ctyrector
Give the set of reactants (including an alkyl halide and a nucleophile) that could be used to synthesize the following ether: draw the molecules on the canvas by choosing buttons from the tools (for bonds and charges), atoms, and templates toolbars, including charges where needed. ch3ch2och2ch2chch3 | ch3
Answers: 1


Chemistry, 22.06.2019 17:00, smelcher3900
According to the kinetic-molecular theory, what happens to a liquid when it is transferred from one container to another? the volume and the shape stay the same. the volume increases to fill the new container, but the shape stays the same. the volume stays the same, but the shape changes to fit the new container. the volume and the shape change to fill the new container.
Answers: 2

Chemistry, 22.06.2019 20:00, aksambo4707
Many free radicals combine to form molecules that do not contain any unpaired electrons. the driving force for the radical–radical combination reaction is the formation of a new electron‑pair bond. consider the chemical equation. n(g)+no(g)⟶nno(g) n(g)+no(g)⟶nno(g) write lewis formulas for the reactant and product species in the chemical equation. include nonbonding electrons. n(g)n(g) select draw rings more erase select draw rings more erase select draw rings more erase n no(g)
Answers: 1
Do you know the correct answer?
CaCO3 decomposes in the equation below. What is the enthalpy of the reaction?
CaCO3 (S) ---> Ca...
Questions in other subjects:





World Languages, 12.08.2020 07:01











