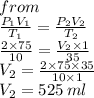Chemistry, 03.05.2021 18:30, katherine3084
3. At 10.0°C and 2.00 atm, the volume of the gas is 75.0 mL. If the temperature of the gas increases to
35.0°C and the pressure decreases to 1.00 atm, what is the new volume?
a. 1630 mL
b. 138 ml
c. 163 mL
d. 0.0017mL

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:00, carvajalj2520
Explain what happens at the saturation point when adding salt to water at room temperature.
Answers: 1

Chemistry, 23.06.2019 02:00, Paytonsmommy09
Butane gas reacts with oxygen gas to give carbon dioxide gas and water vapor (gas). if you mix butane and oxygen in the correct stoichiometric ratio, and if the total pressure of the mixture is 390 mmhg, what is the pressure (in mmhg) of water vapor after the reaction is completed (temperature and volume do not change).
Answers: 2

Chemistry, 23.06.2019 06:50, summerjoiner
What is the volume of 3.2 moles of chlorine gas (cl2) at 295 k and 1.1 atm?
Answers: 1
Do you know the correct answer?
3. At 10.0°C and 2.00 atm, the volume of the gas is 75.0 mL. If the temperature of the gas increases...
Questions in other subjects:

Chemistry, 01.03.2021 20:00




Biology, 01.03.2021 20:00

Mathematics, 01.03.2021 20:00

Arts, 01.03.2021 20:00

Chemistry, 01.03.2021 20:00