2. For the reaction
NH3 + O2 - NO + H2O
a. Balance the equation.
b. What is the...

Chemistry, 03.05.2021 01:00, aaronnnn6998
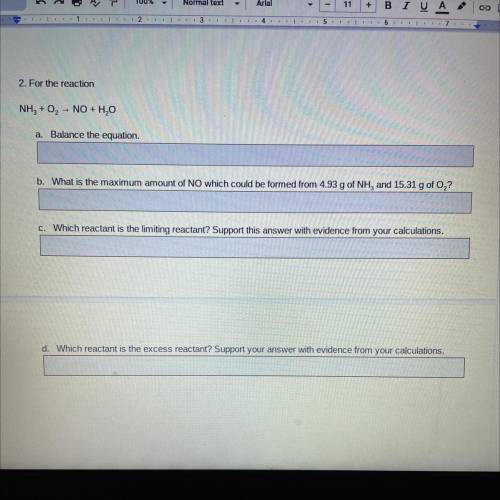
2. For the reaction
NH3 + O2 - NO + H2O
a. Balance the equation.
b. What is the maximum amount of NO which could be formed from 4.93 g of NH, and 15.31 g of O,?
C. Which reactant is the limiting reactant? Support this answer with evidence from your calculations.
d. Which reactant is the excess reactant? Support your answer with evidence from your calculations.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 02:30, brittanysanders
When you perform this reaction, what could remain at the end of the reaction? check all that apply. excess reactant aqueous copper chloride excess reactant aluminum oxygen product solid copper carbon dioxide product aqueous aluminum chloride water
Answers: 2

Do you know the correct answer?
Questions in other subjects:




Biology, 02.10.2019 01:30


History, 02.10.2019 01:30


Geography, 02.10.2019 01:30







