
Chemistry, 02.05.2021 23:40, 2023brewerantonio
A. Write a series of balanced equations, and fill in the blanks for an overall equation, for the complete oxidation of 2 mol of glutamate to 2 mol of α-ketoglutarate and 1 mol of urea. Note that the overall equation (net reaction) does not include aspartate, fumarate, or oxaloacetate, so you must include balanced equations for the interconversion of these molecules. (Hint: what pathway converts fumarate to oxaloacetate?) For reference, the urea cycle figure is shown on the back. You will need a total of 9 to 11 reactions in order to generate the balanced overall equation.
Overall equation/net reaction (fill in stoichiometry):(The stoichiometry of water and H+is not important here, so don’t stress about that)2 glutamate + __CO2+ 4 H2O + __NAD++ __ATP →2 α-ketoglutarate + urea + ___NADH + 7 H++ __ADP + __AMP + __Pi+ __PPi
b. Based on the final equation above, does the process result in a net gain or loss of ATP equivalents? How many?
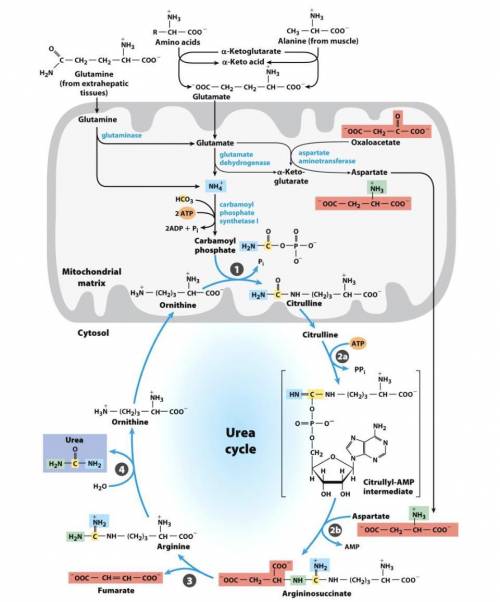

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 20:00, ahnorthcutt4965
Acm ruler with main graduations from 1 to 10 from left to right there are 10 secondary graduations between each of the main graduations there is a line that begins. at the left end of the ruler 10 secondary graduations to the left of the “1 main graduation the right end of the line ends on the eighth secondary graduation to the right of 3 how long is the line
Answers: 1


Chemistry, 22.06.2019 22:30, kiera2599
3.09 lab: reaction of metals 1 which combinations of substances resulted in a chemical change? for each metal that participated in a chemical change, write the type of metal it is, based on your examination of the periodic table. were there any metallic compounds that did not react with either the acid or the base? write the type of metal, based on your examination of the periodic table. make a general statement about the reactivity of the metals in this experiment.
Answers: 1
Do you know the correct answer?
A. Write a series of balanced equations, and fill in the blanks for an overall equation, for the com...
Questions in other subjects:

Biology, 01.08.2019 12:30

History, 01.08.2019 12:30

Mathematics, 01.08.2019 12:30

Biology, 01.08.2019 12:30




Mathematics, 01.08.2019 12:30







