
Chemistry, 02.05.2021 01:00, lilzaya510
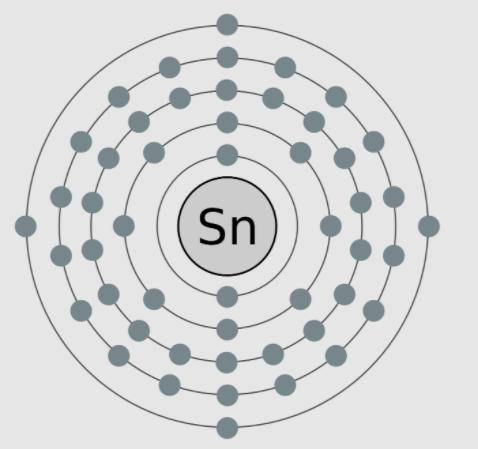
A model of tin, an element with the atomic number 50, is shown here. The valence electrons are modeled here in this image. Which statements are supported by the information in the model? Select ALL That apply.
A) Tin needs four more electrons to complete its outer shell.
B) Tin has no neutrons in the nucleus, as is shown in the model.
C) Tin is highly reactive because it only has four valence electrons.
D) Tin is negatively charged because it more electrons than protons.
E) Tin has a low reactivity because it has full inner shells of electrons.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:30, aedmund1225
Agas has a volume of 0.7 l at 300 mmhg. what would be the new volume at 900 mmhg
Answers: 1

Chemistry, 22.06.2019 00:30, tdowling331
What must happen before a body cell can begin mitotic cell division
Answers: 2

Chemistry, 22.06.2019 12:30, UaRemomGAY
If anyone would be able to me out with these three questions it would be these are from the chem 2202 course.
Answers: 3

Chemistry, 22.06.2019 22:30, lanashanabJHsbd1099
Who discovered a pattern to the elements in 1869?
Answers: 1
Do you know the correct answer?
A model of tin, an element with the atomic number 50, is shown here. The valence electrons are model...
Questions in other subjects:

History, 11.11.2019 20:31






Physics, 11.11.2019 20:31

History, 11.11.2019 20:31







