
Chemistry, 30.04.2021 17:30, janinecastillo01
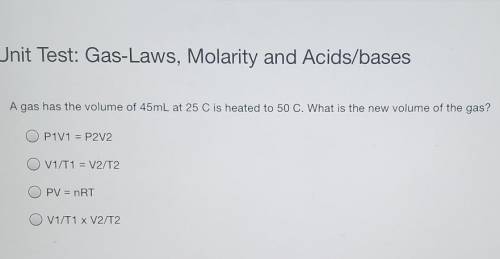
A gas has the volume of 45mL at 25 C is heated to 50 C. What is the new volume of the gas? P1V1 = P2V2 V1/T1 = V2/T2 PV = nRT V1/T1 x V2/T2

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:10, kakesheco4210
What approach is required to balance the objectives of sustainable development? balancing the objectives of sustainable development requires a(n) .
Answers: 3


Chemistry, 22.06.2019 23:00, lufung8627
Consider the reaction: 2al(s) + fe2o3(s) → al2o3(s) + 2fe(s) the δhf for fe2o3(s) = -824.3 kj/mole. the δhf for al2o3(s) = -1675.7 kj/mole. finish the equation. δhrxn = [(1)( kj/mole) + (2)( kj/mole)] - [(1)( kj/mole) + (2) ( kj/mole)]
Answers: 1
Do you know the correct answer?
A gas has the volume of 45mL at 25 C is heated to 50 C. What is the new volume of the gas? P1V1 = P2...
Questions in other subjects:





Mathematics, 02.12.2019 20:31

Mathematics, 02.12.2019 20:31

Mathematics, 02.12.2019 20:31

Mathematics, 02.12.2019 20:31


Mathematics, 02.12.2019 20:31






