
Chemistry, 30.04.2021 15:40, charlesmb7985
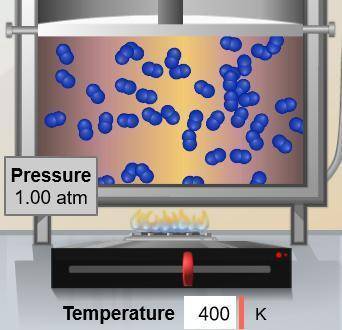
If the volume occupied by the gas molecules shown below were doubled, what would happen to the pressure they exert? (Assume constant temperature.)
A. 0.25 atm
B. 0.50 atm
C. 1.00 atm
D. 2.00 atm

Answers: 3
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 05:30, nuclearfire278
Why is soap used to remove grease? a. its nonpolar end dissolves the grease. b. it makes the water bond with the grease. c. it chemically bonds with the grease. d. its polar end dissolves the grease. correct answer for apex - a, its nonpolar end dissolves the grease.
Answers: 1
Do you know the correct answer?
If the volume occupied by the gas molecules shown below were doubled, what would happen to the press...
Questions in other subjects:



History, 05.09.2020 19:01



Mathematics, 05.09.2020 19:01



Chemistry, 05.09.2020 19:01







