
Chemistry, 29.04.2021 22:30, hunter0156
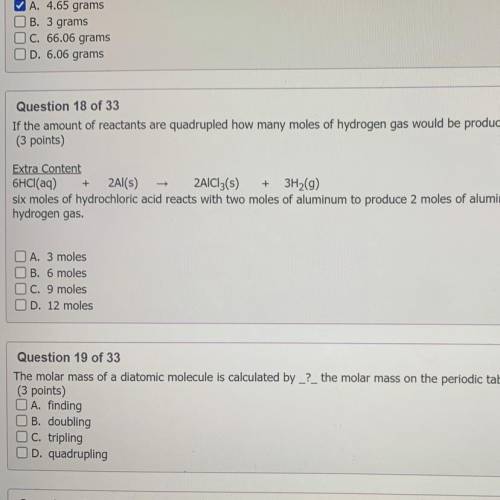
If the amount of reactants are quadrupled how many moles of hydrogen gas would be produced?
(3 points)
+
+
Extra Content
6HCl(aq) 2Al(s) 2AlCl3(5) 3H2(g)
six moles of hydrochloric acid reacts with two moles of aluminum to produce 2 moles of aluminum chloride plus 3 moles of
hydrogen gas.
O A. 3 moles
B. 6 moles
OC. 9 moles
OD. 12 moles

Answers: 1
Other questions on the subject: Chemistry


Do you know the correct answer?
If the amount of reactants are quadrupled how many moles of hydrogen gas would be produced?
(3 poi...
Questions in other subjects:

Mathematics, 02.08.2019 08:10


Mathematics, 02.08.2019 08:10

Mathematics, 02.08.2019 08:10


Mathematics, 02.08.2019 08:10


Chemistry, 02.08.2019 08:10


Social Studies, 02.08.2019 08:10






