Question:
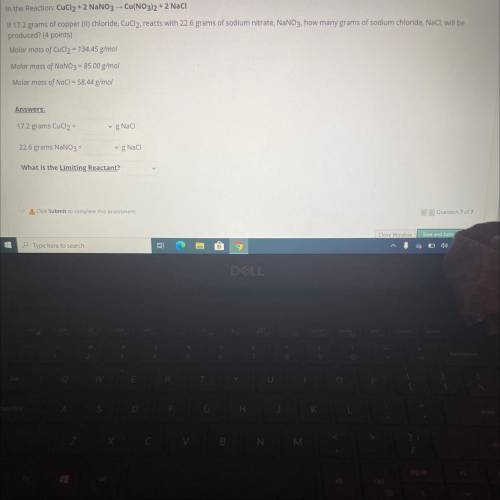
In the Reaction: CuCl2 + 2 NaNO3 -- Cu(NO3)2 + 2 Naci
If 17.2 grams of copper (II)...

Chemistry, 29.04.2021 19:40, quickestlearner8562
Question:
In the Reaction: CuCl2 + 2 NaNO3 -- Cu(NO3)2 + 2 Naci
If 17.2 grams of copper (II) chloride, CuCl2, reacts with 22.6 grams of sodium nitrate, NaNO3. how many grams of sodium chloride, NaCl, will be
produced? (4 points)
Molar mass of CuCl2 = 134.45 g/mol
Molar mass of NaNO3 = 85.00 g/mol
Molar mass of NaCl = 58.44 g/mol
Answers:
17.2 grams CuCl2 =
gNaci
v
22.6 grams NaNO3 =
g Naci
What is the Limiting Reactant?
No

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, mandy9386
You are making a solution of calcium chloride dissolved in water. you add solid, stir, and it dissolves. you add just a spatula tip full, stir, and the solid does not dissolve. how could you describe the solutions before and after adding the spatula tip amount
Answers: 1

Chemistry, 22.06.2019 10:00, 2019reynolds
Which sentence about particles in matter is true? a. atoms are present in solids and liquids but not in gases. b. the particles of matter are in constant motion. c. the same kinds of atoms are found in different elements. d. when a solid changes to a liquid, the sizes of the particles change.
Answers: 1

Chemistry, 22.06.2019 12:10, coastieltp58aeg
Building glycogen from glucose molecules is an example of
Answers: 3

Chemistry, 22.06.2019 16:00, bbrogle5154
If 15 drops of ethanol from a medical dropper weight 0.60g, how many drops does it takes from a dropper to dispense 1.0ml of ethanol? the density of ethanol is 0.80g/ml
Answers: 1
Do you know the correct answer?
Questions in other subjects:

Health, 21.10.2021 08:00


Physics, 21.10.2021 08:00


Mathematics, 21.10.2021 08:00

Biology, 21.10.2021 08:10


Mathematics, 21.10.2021 08:10

Mathematics, 21.10.2021 08:10

Mathematics, 21.10.2021 08:10






