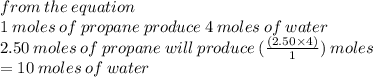C3H8, + 5O2
+ 5O2,3CO2 + 4H20
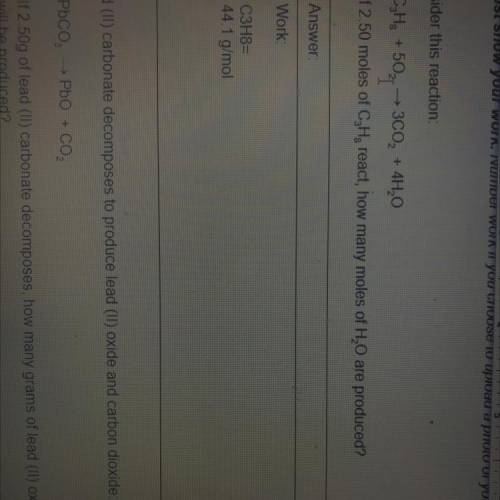
If 2.50 moles of C3H8react, how many moles of H20 are produced...

Chemistry, 28.04.2021 21:30, bevanscory123
C3H8, + 5O2
+ 5O2,3CO2 + 4H20
If 2.50 moles of C3H8react, how many moles of H20 are produced?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 22:10, zwbaby3693
Which aqueous solution of ki freezes at the lowest temperature? 1) 1 mol of ki in 500. g of water 2) 2 mol of ki in 500. g of water 3) 1 mol of ki in 1000. g of water 4) 2 mol of ki in 1000. g of water
Answers: 3

Chemistry, 23.06.2019 00:00, sanaiajohnson56
In an exothermic reaction, energy may be released to the surroundings in the form of question 4 options: heat light thermal all of the above
Answers: 3

Chemistry, 23.06.2019 02:00, Paytonsmommy09
Butane gas reacts with oxygen gas to give carbon dioxide gas and water vapor (gas). if you mix butane and oxygen in the correct stoichiometric ratio, and if the total pressure of the mixture is 390 mmhg, what is the pressure (in mmhg) of water vapor after the reaction is completed (temperature and volume do not change).
Answers: 2

Chemistry, 23.06.2019 04:00, Tiredd7838
Which of these are physical changes in matter? check all that apply boiling water a pencil being sharpened exploding dynamite freezing water rotting cheese
Answers: 1
Do you know the correct answer?
Questions in other subjects:






Mathematics, 17.10.2019 05:40

Mathematics, 17.10.2019 05:40

French, 17.10.2019 05:40