Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:00, pollywallythecat
Your friend offers to show you an intrusive igneous rock. which of the following would you expect to see?
Answers: 1

Chemistry, 21.06.2019 22:30, kingteron5870
Ionic compounds are made of ions, and yet the overall charge of an ionic compound is neutral. why?
Answers: 1

Chemistry, 22.06.2019 04:00, clairebear66
What three natural resources are found in the great lakes region
Answers: 2

Chemistry, 22.06.2019 04:00, eborkins
Seltzer water is created by placing water under pressure with carbon dioxide gas. which of the following statements best describe seltzer water: a. the solution will be slightly acidic b. the solution will be slightly basic. the solution will be strongly acidic. d. the solution will be strongly basic. e. the solution will be neutral
Answers: 3
Do you know the correct answer?
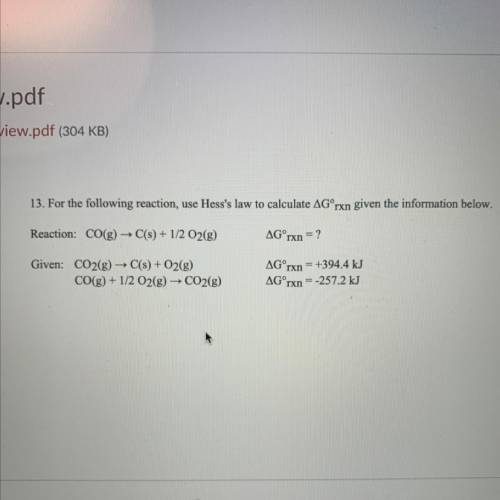
13. For the following reaction, use Hess's law to calculate AGʻrxn given the information below.
Re...
Questions in other subjects:

Mathematics, 28.10.2020 17:40


History, 28.10.2020 17:40


Spanish, 28.10.2020 17:40


Physics, 28.10.2020 17:40


Social Studies, 28.10.2020 17:40