
Chemistry, 28.04.2021 03:00, donaji1024perez
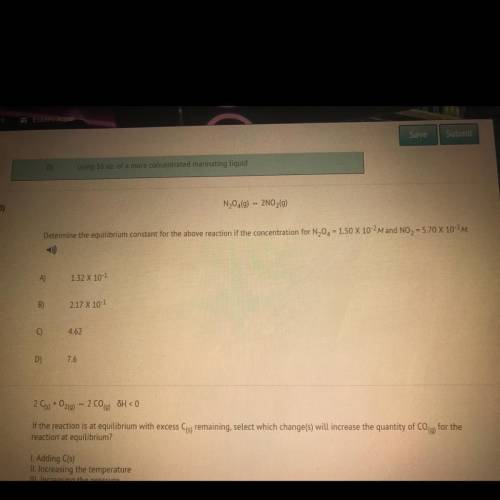
Determine the equilibrium constant for the above reaction if the concentration for N20 = 150 X 10-2M and NO2 = 5.70 X 10-2M.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:30, cxttiemsp021
Calculate the mass of carbon in 97.0 g of sucrose c12h22o11
Answers: 3

Chemistry, 23.06.2019 01:00, MrTeriffic
Imagine if during the cathode ray experiment, the size of the particles of the ray was the same as the size of the atom forming the cathode. which other model or scientific observation would have also been supported? 1. this would support dalton's postulates that proposed the atoms are indivisible because no small particles are involved. 2. this would support bohr's prediction about electrons moving in orbits having specific energy. 3. this would support bohr's prediction about electrons being randomly scattered around the nucleus in the atom. 4. this would support dalton's postulates that proposed that atoms combine in fixed whole number ratios to form compounds.
Answers: 1

Chemistry, 23.06.2019 02:30, ijustneedhelp29
Which of the following statements are incorrect?
Answers: 3

Chemistry, 23.06.2019 07:30, jessicawelch25
In a laboratory determination of the atomic weight of tin, a sample of tin is weighed in a crucible. nitric acid is added, and the reaction proceeds to give a hydrated tin(iv)oxide plus no2and h2o. the hydrated tin(iv)oxide is then heated strongly and reacts as follows: sno2.xh2o(s)sno2(s)+ xh2o(g)the sno2is finally cooled and weighed in the crucible. explain the effect on the calculated atomic weight of tin that would result from each of the following experimental errors: (a)considerable spattering occurs when the nitric acid is added to the tin.(b)the hydrated tin(iv)oxide is not heated sufficiently to change it completely to tin oxide.
Answers: 2
Do you know the correct answer?
Determine the equilibrium constant for the above reaction if the concentration for N20 = 150 X 10-2M...
Questions in other subjects:

Mathematics, 02.01.2020 23:31


Mathematics, 02.01.2020 23:31

Social Studies, 02.01.2020 23:31

Mathematics, 02.01.2020 23:31











