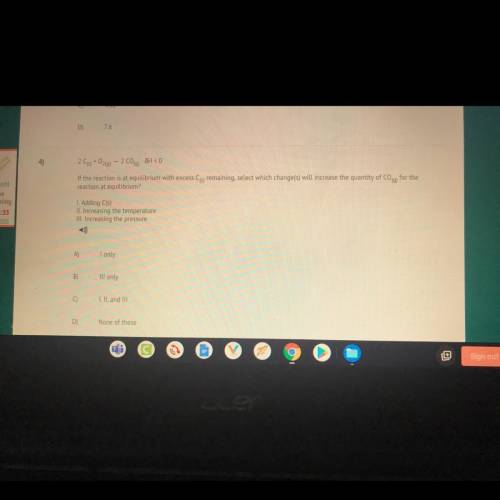
4)
209+0260) - 2 C00) JH < 0
If the reaction is at equilibrium with excess Cs remaining,...

Chemistry, 28.04.2021 02:50, mahagonylabeyta
4)
209+0260) - 2 C00) JH < 0
If the reaction is at equilibrium with excess Cs remaining, select which change(s) will increase the quantity of Coq) for the
reaction at equilibrium?
I. Adding C(s)
II. Increasing the temperature
III. Increasing the pressure

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, darrriannn7241
What is the correct lewis structure for chloroform chcl3
Answers: 1

Chemistry, 22.06.2019 13:00, torigirl4126
In what environment would mineral formation caused by high pressures and high temperatures most likely occur?
Answers: 3

Chemistry, 22.06.2019 18:00, LuvieAnn1886
How is energy related to the change of state represented by the model? atoms gain energy as a solid changes to a liquid. atoms gain energy as a solid changes to a gas. atoms lose energy as a solid changes to a liquid. atoms lose energy as a solid changes to a gas.
Answers: 3

Chemistry, 23.06.2019 06:30, artbydi
Achemist is studying the following equilibirum, which has the given equilibrium constant at a certain temperature: 2 no(g) + cl2(g) < => 2 nocl(g) kp = 2 x 10^(-6)he fills a reaction vessel at this temperature with 13. atm of nitrogen monoxide gas and 12. atm of chlorine gas. use this data to answer the questions: a. can you predict the equilibrium pressure of noci, using only the tools available to you within aleks? y/nb. if you said yes, then enter the equilibrium pressure of nocl at right. round your answer to 1 significant digit.
Answers: 1
Do you know the correct answer?
Questions in other subjects:



Social Studies, 25.07.2019 06:00


Social Studies, 25.07.2019 06:00




Mathematics, 25.07.2019 06:00






