
Chemistry, 27.04.2021 18:20, darcyshay62871
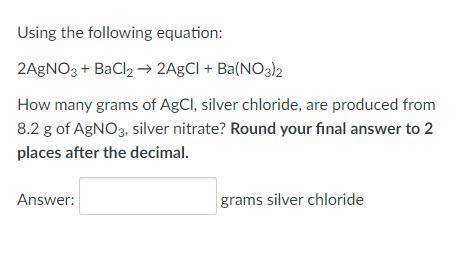
Using the following equation:
2AgNO3 + BaCl2 → 2AgCl + Ba(NO3)2
How many grams of AgCl, silver chloride, are produced from 8.2 g of AgNO3, silver nitrate? Round your final answer to 2 places after the decimal.
_grams silver chloride

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 08:30, Blaise2653
Joan writes four numbers on the board in standard form, and then she writes their scientific notation
Answers: 1

Do you know the correct answer?
Using the following equation:
2AgNO3 + BaCl2 → 2AgCl + Ba(NO3)2
How many grams of AgCl...
How many grams of AgCl...
Questions in other subjects:

Mathematics, 17.12.2020 22:10


Mathematics, 17.12.2020 22:10

Mathematics, 17.12.2020 22:10

History, 17.12.2020 22:10

Geography, 17.12.2020 22:10

Mathematics, 17.12.2020 22:10



Mathematics, 17.12.2020 22:10






