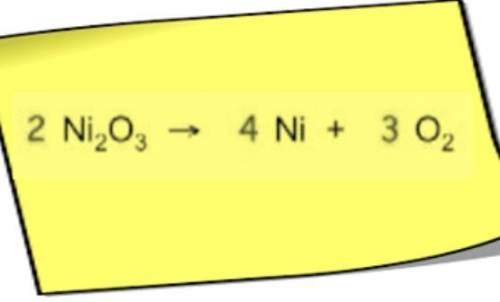
Chemistry, 27.04.2021 15:20, karena13aguirre
Your advisor has given you an unknown isotope and asked you to identify it. You see fromher notes that the mass of the isotope is 228.03107 u, the atomic mass number is 228, andthat it has a total binding energy of 1742.43 MeV.(A) Find the sum of the masses of the components that make up the neutral atom. Hint:Recall that the binding energy is the difference between the rest energy of the piecesthat make up an atom and the rest energy of the whole atom when those pieces havecome together.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:00, berniceallonce22
What is the atomic mass of an atom that has 6 protons, 6 neutrons, and 6 electrons? a) 6 b) 8 c) + 1 d) 12 e) 18
Answers: 1


Chemistry, 22.06.2019 13:30, bryce99
In a ni-cd battery, a fully charged cell is composed of nickelic hydroxide. nickel is an element that has multiple oxidation states. assume the following proportions of the states: nickel charge proportions found 0 0.17 +2 0.3 +3 0.33 +4 0.5 (a) determine the mean of the nickel charge. enter the answer to 2 decimal places.(b) determine the cumulative distribution function of nickel charge.
Answers: 2

Chemistry, 22.06.2019 14:50, ladybugperez05
Which of the following is most likely true about water in chemical systems? a) water dissolves nonpolar ionic compounds. b) water dissociates ionic compounds. c) water dissociates covalent molecules. d) water dissolves nonpolar covalent substances.
Answers: 1
Do you know the correct answer?
Your advisor has given you an unknown isotope and asked you to identify it. You see fromher notes th...
Questions in other subjects:



History, 17.03.2022 18:10




Mathematics, 17.03.2022 18:10

Mathematics, 17.03.2022 18:10