Please help
The thermochemical equation for the combustion of propane gas is:
CH4 (g)...

Chemistry, 27.04.2021 02:10, coolgirl5679
Please help
The thermochemical equation for the combustion of propane gas is:
CH4 (g) + 2O2 (g) CO2 (g) + 2H2O (I), ΔH = -890 kJ/mol
Calculate much heat is released when 3.5 moles of propane have a combustion reaction.
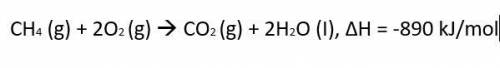

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:30, sotoamerica0814
98 ! and brainliest plz ! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 15:00, hockeykid7583
Which are forms of frozen water? check all that apply. dew frost hail rain sleet
Answers: 2

Chemistry, 22.06.2019 16:30, montanolumpuy
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a. 3.4 mol h2so4b. 6.8 mol h2so4 c. 10.2 mol h2so4 d. 13.6 mol h2so4
Answers: 3

Chemistry, 22.06.2019 22:30, jkjjoijjm5928
Akno3 solution containing 51 g of kno3 per 100.0 g of water is cooled from 40 ∘c to 0 ∘c. what will happen during cooling?
Answers: 3
Do you know the correct answer?
Questions in other subjects:

Computers and Technology, 22.01.2020 06:32







Business, 22.01.2020 06:32

Business, 22.01.2020 06:32







